1. Which of the following is not an organic molecule?
a. cellulose
b. sucrose
c. water
d. testosterone
c.Water
2. Which of the following terms includes all the other terms on this list?
a. polysaccharide
b. carbohydrate
c. monosaccharide
d. glycogen
a. Polysaccharide
3. Which term is most appropriate to describe a molecule that dissolves easily in water?
a. hydrocarbon
b. hydrophobic
c. hydrophilic
d. organic
c. Hydrophilic
4. Cholesterol is an example of what kind of molecule?
a. protein
b. lipid
c. amino acid
d. carbohydrate
b. Lipid
5. The 20 amino acids vary only in their
a. carboxyl groups
b. side groups
c. amino groups
d. lipid groups
b. Side groups
6. A specific reactant an enzyme acts upon is called the
a. catalyst
b. sucrase
c. active site
d. substrate
d. Substrate
7. An enzyme does which of the following?
a. adds heat to a reaction, speeding it up
b. lowers the activation energy of a reaction
c. cools a reaction, slowing it down
d. raises the activation energy of a reaction
b. Lowers the activation energy of a reaction
8. Besides satisfying your hunger, why else might you consume a big bowl of pasta the night before a race?
Since pasta has plenty of carbohydrates in it, it is useful to an athlete as it contains lots of sugar.
9. How are glucose, sucrose, and starch related?
Glucose, sucrose, and starch are all related as they are all sugars. They all have the same ratio of carbon to hydrogen to oxygen (1:2:1, respectively)
10. What are steroids? Describe two functions they have in cells
Steroids are lipids with fused rings of carbon. Two functions they have in cells are being in the cell membranes and protecting the cell (cholesterol), and making sex cells (testosterone and estrogen).
11. How are polypeptides related to proteins?
Polypeptides are the long chains formed by amino acids that make up proteins.
12. How does denautration affect the ability of a protein to function?
When a protein denaturates, it unravels and breaks down, rendering it useless.
14. The reaction below (top of page 107) shows two amino acids joining together.
a. One product of this reaction is represented by a question mark. Which molecule is it?
H2O, or water.
b. What is this kind of reaction called? Explain.
This was a dehydration reaction as it lost a water molecule in the joining of the two amino acids.
c. If an amino acid were added to this chain, at what two places could it attach?
Another amino acid could be added at either of the two OHs on the bottom right.
15. Use the graph (middle of page 107) to answer the questions below.
a. At which temperature does enzyme A perform best? Enzyme B?
Enzyme A would perform best at about 38 degrees Celsius, while Enzyme B would perform best at about 78 degrees Celsius.
b. Knowing that one of these enzymes is found in humans and the other in thermophilic (heat-loving) bacteria, hypothesize which enzyme came from which organism?
I think that Enzyme A is from a human body, and Enzyme B is from the thermophilic bacteria because if the bacteria is thermophilic, it must be able to function in higher heats than human enzymes most likely would.
c. Propose a hypothesis that explains why the rate of the reaction catalyzed by enzyme A slows down at temperatures above 40 degrees Celsius.
I think that Enzyme A slows down at temperatures about 40 degrees Celsius because it is simply not designed to function under temperatures that humans could not survive in anyways.
Tuesday, September 9, 2008
Friday, September 5, 2008
Summary of Concept 5.5
Concept 5.5
Notes
1. Explain the role of activation energy in a reaction. How does an enzyme affect activation energy?
Activation energy is the energy required to be inputted for the reaction to occur, otherwise reactions would all occur spontaneously. Enzymes affect activation energy by bringing down the required amount of energy for the reaction to occur.
2. Describe how a substrate interacts with an enzyme.
Subtrates fit inside the part of the enzyme called the active energy, and the enzyme allows it to cause the chemical reaction in a faster manner, through decreasing the amount of activation energy required.
Notes
- All chemical reactions require activation energy to be put in for the for the reaction to occur.
- Activation energy is often provided through heat.
- Catalysts are compounds that speed up chemical reactions.
- Enzymes are specialized proteins that work as catalysts on an organic scale.
- Catalysts work by reducing the required activation energy.
- Enzymes only catalyze one specific kind of reaction because enzymes only fit the shape of one reactant molecule.
- The reactant that enzymes catalyze are called substrates, which go inside the region of the enzyme molecule called the active site.
- Temperature and pH affect the effectiveness of an enzyme.
1. Explain the role of activation energy in a reaction. How does an enzyme affect activation energy?
Activation energy is the energy required to be inputted for the reaction to occur, otherwise reactions would all occur spontaneously. Enzymes affect activation energy by bringing down the required amount of energy for the reaction to occur.
2. Describe how a substrate interacts with an enzyme.
Subtrates fit inside the part of the enzyme called the active energy, and the enzyme allows it to cause the chemical reaction in a faster manner, through decreasing the amount of activation energy required.
Summary of Concept 5.4
Concept 5.4
Notes
1. Give at least two examples of a protein you can "see" in the world around you. What are their functions?
Nuts have many proteins in them, and they feed animals such as chipmunks and squirrels. Muscles also have protein in them, resulting in animals being able to move (and being able to provide nutritious protein through their meat).
2. Relate amino acids, polypeptides, and proteins.
Amino acids are the monomers that make up polypeptides, polypeptides make up proteins, and proteins make up organisms.
3. Explain how heat can destroy a protein.
Heat can destroy a protein through the process of denaturation, in which the protein unravels due to outside environmental changes (in this case, heat). It unfolds the proteins' polypeptides, meaning the protein is no longer a protein, but rather, several groups of polypeptides.
4. Which parts of an amino acid's structure are the same in all amino acids? What part is unique?
In all amino acids, there is a carbon atom in the middle of the molecule, but what surrounds it is what makes amino acids different from one another.
Notes
- Proteins are polymer constructed from monomers called amino acids.
- Amino acids are monomers made of one carbon bonded to four other groups.
- Polypeptides are amino acid chains.
- There are 20 different kinds of amino acids, and can be combined in many different ways to form different proteins.
- Proteins are put together in special ways, therefore resulting in different shapes of proteins.
- Hydrophilic amino acids stay on the outside of proteins, while hydrophobic amino acids stay on the inside.
- Protein denaturates (loses its shape due to a change in temperature, pH, or other environmental qualities).
1. Give at least two examples of a protein you can "see" in the world around you. What are their functions?
Nuts have many proteins in them, and they feed animals such as chipmunks and squirrels. Muscles also have protein in them, resulting in animals being able to move (and being able to provide nutritious protein through their meat).
2. Relate amino acids, polypeptides, and proteins.
Amino acids are the monomers that make up polypeptides, polypeptides make up proteins, and proteins make up organisms.
3. Explain how heat can destroy a protein.
Heat can destroy a protein through the process of denaturation, in which the protein unravels due to outside environmental changes (in this case, heat). It unfolds the proteins' polypeptides, meaning the protein is no longer a protein, but rather, several groups of polypeptides.
4. Which parts of an amino acid's structure are the same in all amino acids? What part is unique?
In all amino acids, there is a carbon atom in the middle of the molecule, but what surrounds it is what makes amino acids different from one another.
Summary of Concept 5.3
Concept 5.3
Notes
1. What properties do lipids share?
All lipids are hydrophobic, meaning they avoid water (like the plague).
2. What are the parts of a fat molecule?
Fat molecules are made of a three carbon skeleton, surrounded by glycerol, with three long chains of acidic hydrocarbon attached.
3. Describe two ways that steroids are different from fats.
Steroids are in a formation of a ring, not a chain, also, steroids have different functions from fats.
4. What does the term unsaturated fat on a food label mean?
When a food label has unsaturated fat written on it, it is generally better for one's body then saturated fat, which has as many hydrogen atoms as possible, as opposed to the unsaturation of hydrogen atoms in unsaturated fats.
Notes
- Oil is in the class of lipids, meaning it avoids water.
- Any molecule that avoids water is hydrophobic.
- Fat is a three carbon backbone with glycerol attached.
- Fats provide your body with insulation.
- Saturated fats have all three acids chains containing as many hydrogen atoms as possible.
- Unsaturated fats are not saturated with hydrogen atoms.
- Too much saturated fat is unhealthy.
- A lipids that have a carbon skeleton forming four fused rings are called steroids.
- Steroids all have different functions.
- Cholesterol is probably the most well known steroid. It is required in the cell membranes of the human body.
1. What properties do lipids share?
All lipids are hydrophobic, meaning they avoid water (like the plague).
2. What are the parts of a fat molecule?
Fat molecules are made of a three carbon skeleton, surrounded by glycerol, with three long chains of acidic hydrocarbon attached.
3. Describe two ways that steroids are different from fats.
Steroids are in a formation of a ring, not a chain, also, steroids have different functions from fats.
4. What does the term unsaturated fat on a food label mean?
When a food label has unsaturated fat written on it, it is generally better for one's body then saturated fat, which has as many hydrogen atoms as possible, as opposed to the unsaturation of hydrogen atoms in unsaturated fats.
Monday, September 1, 2008
Summary of Concept 5.2
Concept 5.2
Notes
1. Explain the difference between a monosaccharide and a disaccharide. Give an example of each.
Monosaccharides are sugars with only one unit, and encompasses things like candy or fruits. Dissacharides are sugars that are made of two monosaccharides, and make up things like milk sugar (lactose) and cane sugar.
2. Compare and contrast starch, glycogen, and cellulose.
While all of the above are polysaccharides, they all come from different organisms. Starch is found in plant cells and is entirely composed of long chains of glucose monomers. Glycogen is a polysaccharide that animals store excess sugar with. And cellulose is similar to starch in that it is entirely formed of glucose, and it protects the cells of a plant, preventing it from entirely flopping over.
3. How do animals store excess glucose molecules?
Animals store excess glucose molecules by turning it into the polymer called glycogen. When they require energy, glycogen is broken down into the more simple glucose.
Notes
- Discusses primarily about the various types of sugars and they're applications in life.
- Carbohydrates are organic compounds made of sugar molecules.
- All sugar molecules have the ratio of 1 Carbon: 2 Hydrogen: 1 Oxygen, and is a various multiple of CH2O.
- One kind of sugars are called monosaccharides, and they only contain one unit of sugar.
- Glucose and Fructose are both monosaccharides and can often be found in sweet things we eat such as candy and honey.
- Our body needs monosaccharides in order to burn into energy. Another kind of sugars are disaccharides.
- Dissacharides link fructose molecules and glucose together.
- Examples of disaccharies are table sugars that come from plants such as beets and sugarcane.
- Our body uses dissacharides to store glucose for later use.
- The last kind of sugars are called polysaccharides, or complex carbohydrates, and they are long polymer chains created from simple sugar monomers.
- Starch is a good example of polysaccharides. Starch is found in plant cells, and in many plants that we eat every day.
- Animal cells do not have starch, but rather a different polysaccharide called glycogen.
- We humans store glycogen in our livers and break it down when we need glucose.
- All carbohydrates are hyrophilic as they all have the hydroxyl group contained in them.
1. Explain the difference between a monosaccharide and a disaccharide. Give an example of each.
Monosaccharides are sugars with only one unit, and encompasses things like candy or fruits. Dissacharides are sugars that are made of two monosaccharides, and make up things like milk sugar (lactose) and cane sugar.
2. Compare and contrast starch, glycogen, and cellulose.
While all of the above are polysaccharides, they all come from different organisms. Starch is found in plant cells and is entirely composed of long chains of glucose monomers. Glycogen is a polysaccharide that animals store excess sugar with. And cellulose is similar to starch in that it is entirely formed of glucose, and it protects the cells of a plant, preventing it from entirely flopping over.
3. How do animals store excess glucose molecules?
Animals store excess glucose molecules by turning it into the polymer called glycogen. When they require energy, glycogen is broken down into the more simple glucose.
Summary of Concept 5.1
Concept 5.1
Notes

2. Explain the connection between monomers and polymers.
Monomers are the molecules that act as the building blocks of polymers, bonding into long chains in order to create complex polymers.
Notes
- Carbon forms bonds with other carbon atoms to form carbon skeletons.
- Organic molecules are carbon based molecules.
- Inorganic molecules are molecules that do not have carbon in them.
- Functional groups are molecules that react in a predictable way with other atoms.
- Examples of function groups include hydroxyl, carbonyl, carboxyl, and amino.
- Hydroxyl is hydrophilic.
- Small molecules are building blocks called monomers.
- Polymers are large molecules made from long chains of monomers.
- Water is released when a monomer is added to a polymer chain.
- Water is required when a monomer is removed from a polymer chain.
Concept Check
1. Draw a molecule that has a three-carbon skeleton and a hydroxyl group on the middle carbon. (Hint: The molecule formula is C3H8O)

2. Explain the connection between monomers and polymers.
Monomers are the molecules that act as the building blocks of polymers, bonding into long chains in order to create complex polymers.
3. What molecule is released during construction of a polymer? What is this reaction called?
The molecule of water (H2O) is released in the construction of a polymer. This reaction is called a hydrolysis reaction.
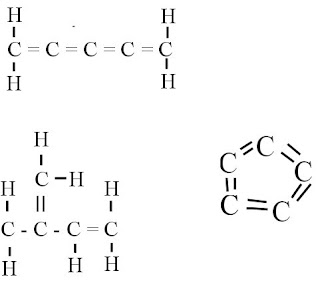
4. Draw at least three ways in which five carbon atoms could be joined to make different carbon skeletons.
Subscribe to:
Posts (Atom)